









|


|
 |
June 2001 : Additional Resources
|
|
 |
Cloud Chamber "How-To" Tips
|
|
 |
What You Need
|
 |
Cloud Chamber Container - this can be a commercially supplied kit OR you can create your own using a deep petri dish with a cover and some blotter paper. Be sure the bottom of the chamber is black, otherwise you'll have difficulty seeing the vapor trails.
Radioactive Source - you can use a commercially supplied source, a piece of red-orange Fiestaware, uranium ore, a lantern mantle containing thorium, etc.
Flashlight - or other light source
Dry Ice - remember that dry ice is VERY cold and can quickly damage tissue; handle with gloves; caution students about hazards!
Alcohol - ethyl preferred, 95% if available; isopropyl not recommended
Cardboard or Paper Plate - to hold the dry ice
Magnet - optional
|
|
 |
Setting Up the Cloud Chamber
|
 |
Some science suppliers offer a cloud chamber source, which has a tiny radioactive sample mounted in the eye of a needle. The source is packed with the needle sticking in a cork or stopper. The cork or stopper fits into an opening in the cloud chamber, sealing it.
|

|
 |
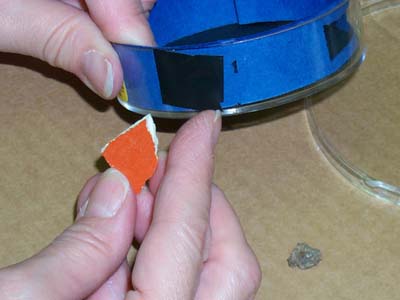
If you are using an ore sample, a piece of Fiestaware, a lantern mantle containing thorium, or some other radioactive source, and the cloud chamber has a hole in it, you will need to seal the hole so that the alcohol vapor can not escape.
|
|
 |
Saturate the blotter paper (or heavy construction paper or felt) with alcohol. You want to have plenty of alcohol, so that the vapor will fill the chamber.
|

|
 |
Insert your radioactive source.
|



|
 |
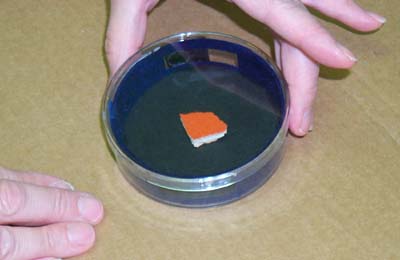
Cover the container tightly so that the alcohol vapor is trapped inside the chamber.
|
|
 |
Place the container on dry ice, making sure that the entire bottom is in contact with the dry ice. The bottom of the container should be as close to level as possible.
|

|
 |
Darken the room. Place a flashlight at the side of the dish, shining the light through the dish. Small adjustments in the angle of the light will make significant changes in what you can see. Experiment with changing the light position.
|



|
 |
As the chamber cools, you should begin to detect vapor trails caused by the motion of the radioactive particles and presence of the rays. Some of the trails will be about one-half inch long. These are caused by alpha particles. Some trails will be longer and thinner (beta).Occasionally you will see faint, twisting or circling tracks (caused by gamma).
|

|
|
 |
Troubleshooting Tips
|
 |
- Sometimes a "stubborn" or non-functioning chamber can be coaxed into action by warming the top with your hand, causing the evaporation of more alcohol. In some cases, you may need to re-saturate the blotter or felt, and start over.
- Patience is sometimes required so that the air and vapor in the container gets cold enough to condense.
- If the chamber has been working well and then the tracks become faint, you might revive its action by rubbing the top with a cotton or silk cloth. The static electricity created this way sometimes helps "clear" the chamber and makes the tracks visible again.
- Some commercially available cloud chambers are supplied with a thick black paper in the bottom. While photographing chambers for this "how-to" piece, we found that chambers with this thick material took far longer to begin functioning than those with plastic bottoms that were painted black. The thick paper may have acted as an insulation that slowed cooling of the chamber. Our suggestion is that you use the thinnest layer of black paper possible, or try painting the bottom of the chamber black.
- It is a good idea to test your equipment before putting students to work on an activity like this! Hands-on experience is invaluable. Still, you should be prepared for the possibility that one lab group will be unable to get the cloud chamber to work. This is a great opportunity to discuss the value of multiple trials as we gather data.
- Humidity and weather conditions can affect functioning of cloud chambers.
- While 70% ethyl alcohol will work, using 95% ethyl alcohol enhances operation. Isopropyl alcohol seems less effective.
|
|
 |
Other Ideas for Students to Try
|
 |
Try measuring how far the alpha and beta particles travel in the cloud chamber. Which travels further?
Hold the north end of a strong magnet near the chamber. Does it appear to have any effect on how the alpha or beta particles move?
Try wrapping the source (uranium ore or Fiestaware, for example) in a piece of paper. What types of radiation are still visible?
Try wrapping the source in aluminum foil. How does this affect the types of radiation tracks you can see?
|
|
|
 |



